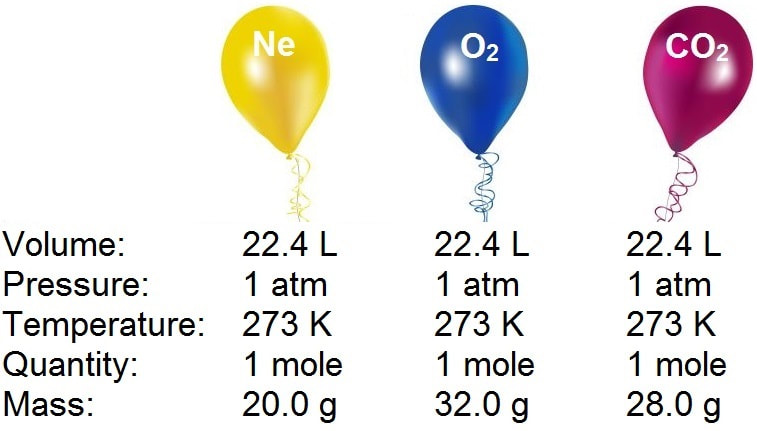
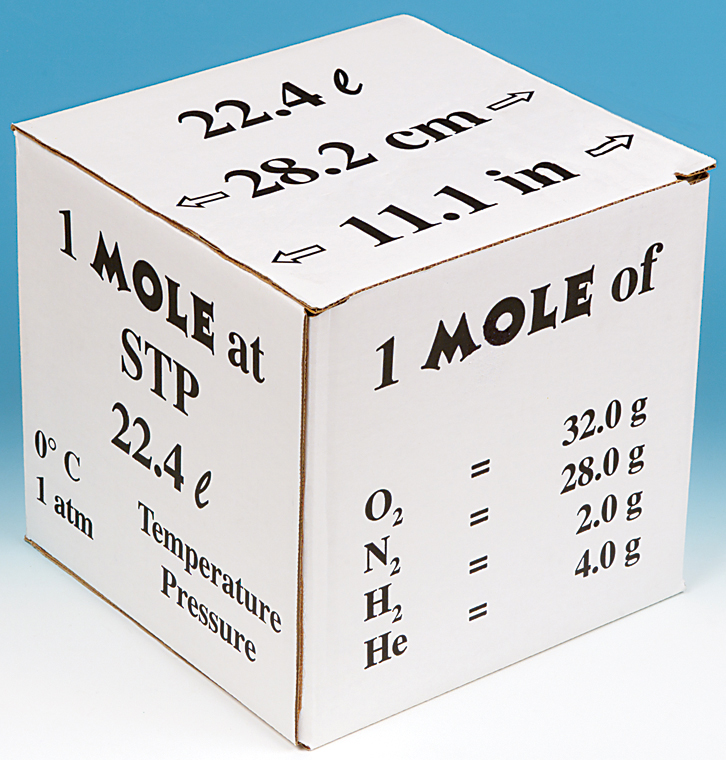
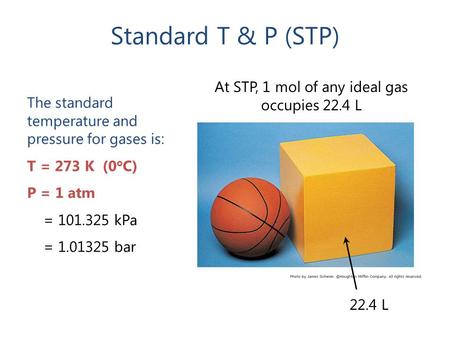
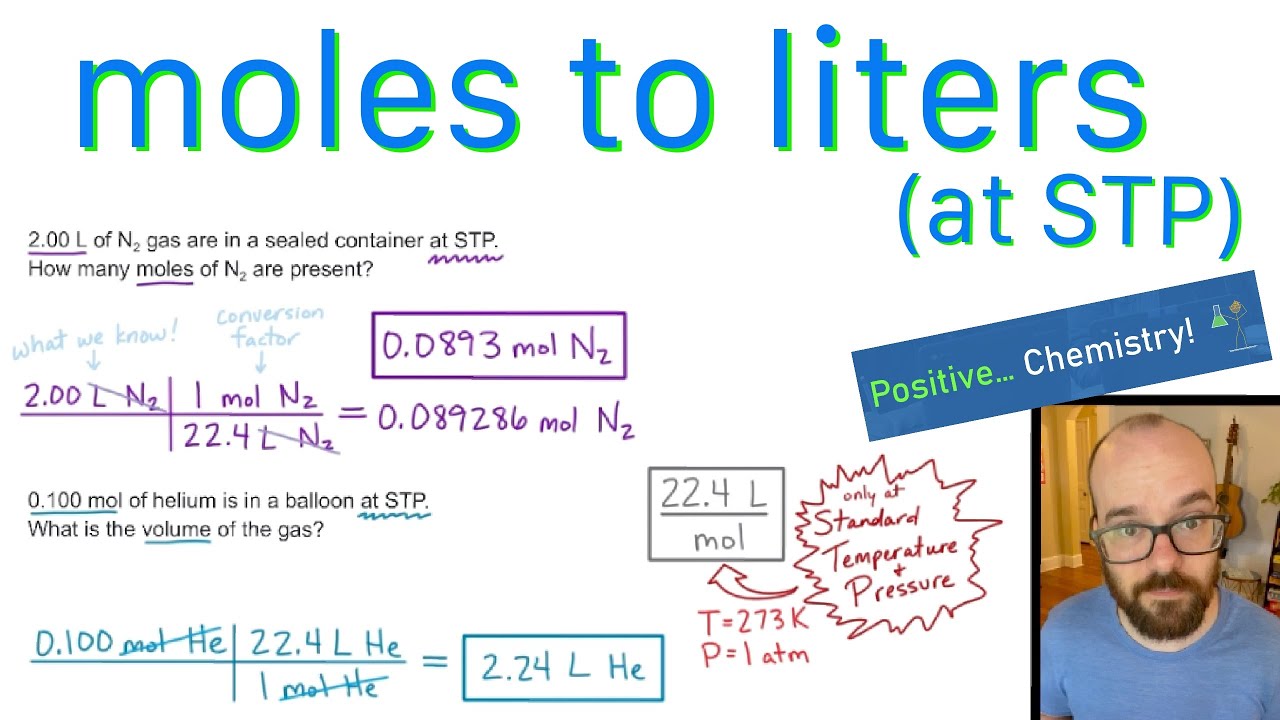
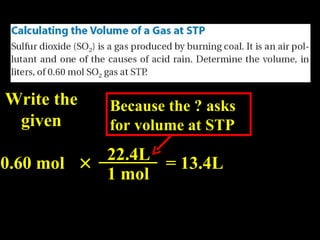
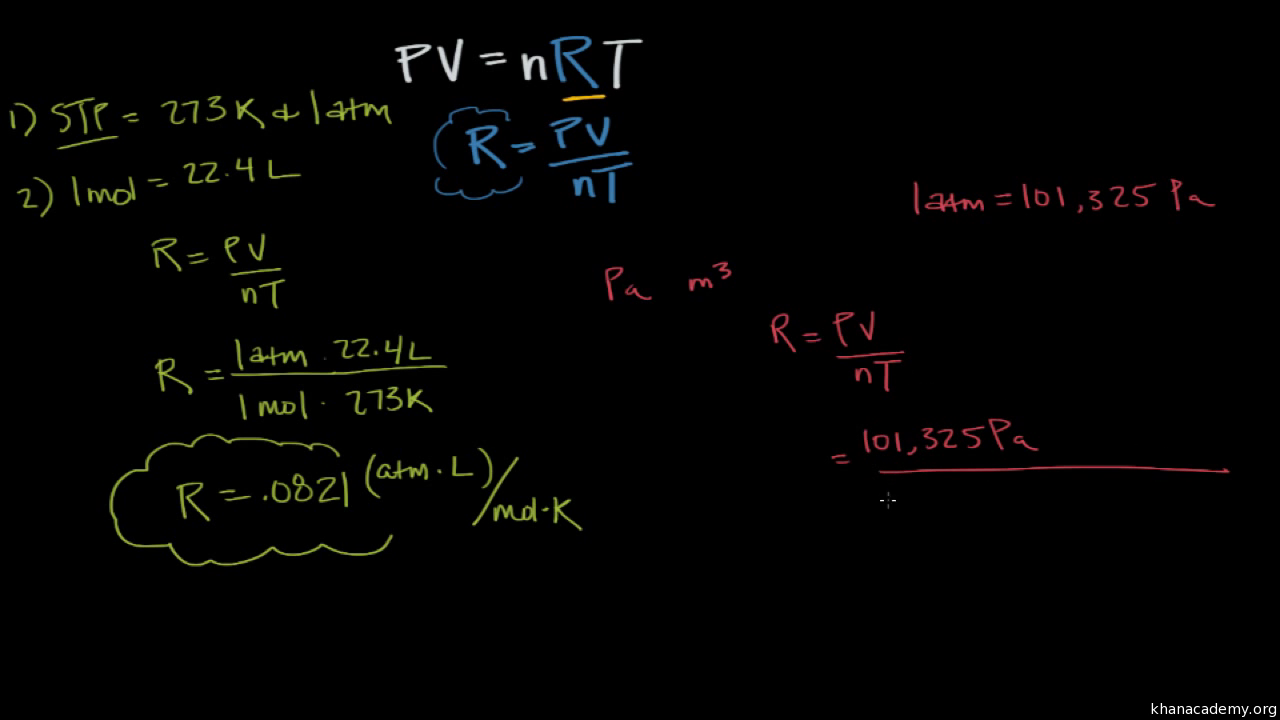
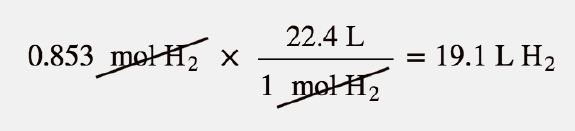Gases & Stoichiometry. Molar Volume 1 mol of gas = 22.4 L molar volume What volume would be occupied by 0.77 moles of helium gas at STP? - ppt download
When 22.4 litres of H2(g) is mixed with 11.2 liters of Cl2(g), each at S.T.P the moles of HCl(g) formed - Sarthaks eConnect | Largest Online Education Community

Gases & Stoichiometry. Molar Volume 1 mol of gas = 22.4 L molar volume What volume would be occupied by 0.77 moles of helium gas at STP? - ppt download